Effect of Caffeine on Serotonin Levels Literature Review
- Research
- Open Access
- Published:
Assessment of the antidepressant effect of caffeine using rat model of depression induced past reserpine
Bulletin of the National Research Center book 42, Commodity number:36 (2018) Cite this article
Abstract
Objective
The present objective is to evaluate the antidepressant action of caffeine.
Material and methods
3 groups of rats were used; control, reserpine-induced rat model of depression, and rat model of low treated daily with caffeine. At the end of the experiment, the motor activity of rats was measured using open field test. On the next day, the animals of the three groups were sacrificed to measure levels of serotonin, norepinephrine, and dopamine in the cortex and hippocampus by spectrofluorometer. In addition, the levels of lipid peroxidation (MDA), nitric oxide (NO), and reduced glutathione (GSH) together with the activities of acetylcholinesterase (AchE) and Na+, K+, ATPase were measured in the two studied brain regions past spectrophotometer.
Results
In the rat model of low, the animals showed a meaning decrease in motor activeness. This was associated with significant decreases in serotonin, norepinephrine, and dopamine in the cortex and hippocampus. However, significant increases in the activities of Anguish and Na+, K+, ATPase, and the levels of MDA and NO were recorded in both areas of rat model of depression while GSH showed a significant subtract in the hippocampus. Caffeine failed to restore the decrease in motor activity. Caffeine treatment ameliorated the changes in cortical and hippocampal norepinephrine and dopamine and hippocampal serotonin. In addition, it restored MDA and GSH levels. However, it failed to prevent the increased AchE and Na+, Thousand+, ATPase activities, and NO levels.
Conclusions
The nowadays findings bespeak that caffeine has a partial antidepressant event mediated by its antioxidant activity and enhancement of monoamine levels.
Groundwork
Caffeine (1,3,7-trimethylxanthine) is a psychoactive agent that is used worldwide in different forms. Due to its lipophilicity, caffeine crosses the blood-brain barrier easily exerting its stimulant issue (Ferré 2016). Caffeine exerts its activity on the central nervous system (CNS) by counteracting near of the inhibitory effects of adenosine on neuroexcitability (Fredholm et al. 1999), arousal (Porkka-Heiskanen 1999), and spontaneous action (Kuzmin et al. 2006). Likewise, the changes induced by caffeine in CNS functions were mediated by reducing phosphodiesterase activity, blocking GABA-A receptor activity, and increasing intracellular calcium (Garrett and Griffiths 1997). The stimulatory effect of caffeine has been attributed to its antagonistic effect on the activeness of endogenous adenosine inhibiting the release of several CNS neurotransmitter systems including γ-aminobutyric acrid (GABA), acetylcholine, glutamate, dopamine, norepinephrine, and serotonin (El Yacoubi et al. 2001; Ferré et al. 1996a; Ferré et al. 1996b; Fisone et al. 2004; Williams 1987) and thus the result of caffeine on low has been taken into consideration.
The preclinical and clinical findings indicate that in that location is a shut human relationship between the adenosine modulatory system and depressive-like behavior (Blardi et al. 2005). Nether normal physiological conditions, adenosine acts as a neuromodulator in the CNS through activating adenosine receptors Ai and A2A in the brain resulting in inhibition of synaptic activity and neurotransmitter release (Fredholm et al. 2005). Information technology has been observed that methylxanthines including caffeine could cake these adenosine receptors (Fredholm et al., 1999).
Depression is an important public health issue. By 2030, it is expected that depression will be one of the tertiary disorders contributing to the universal disease burden (Mathers and Loncar 2006). According to the current World Wellness Organization (WHO) estimation, the number of people suffering from depressive disorders worldwide is 350 million. In addition, WHO predicts that low volition exist the second civilization disorder producing incapacity by 2020.
In that location are controversial data on the relation between the consumption of caffeine and depression. Several epidemiologic studies have found a connection betwixt coffee or caffeine intake and depression, merely the results of existing literature have yielded inconsistent results. Some studies discovered an inverse relationship betwixt java or caffeine and depression (Guo et al. 2014; Pham et al. 2014). Recently, Wang et al. (2016) in a meta-assay of observational studies found that coffee and caffeine intake were significantly linked with reduced risk of depression. Other studies did not discover whatsoever beneficial effect for java and caffeine on low (Pham et al. 2014; Ruusunen et al. 2010). On the other hand, the study of Jin et al. (2016) ended that caffeine intake was positively related to severity of low and insomnia among Korean adolescents.
According to the monoamine hypothesis of depression, the brain depletion of serotonin, norepinephrine, and/or dopamine could underlie the etiology and pathogenesis of depression (Schechter et al. 2005). In improver, several lines of testify indicate the interest of oxidative and nitrosative stress in the pathophysiology of depressive disorders (Maes et al. 2011).
Therefore, the current study aims to evaluate the effect of caffeine on the behavioral (open field exam) and neurochemical (the levels of serotonin, norepinephrine, dopamine, lipid peroxidation, nitric oxide, and reduced glutathione and the activities of acetylcholinesterase and Na+, K+, ATPase) changes occurring in the hippocampus and cerebral cortex of depressive-like rats induced past reserpine.
Material and methods
Animals
Twenty-four male Wistar rats, weighing between 230 and 250 g, were obtained from the Brute Firm of the National Enquiry Centre, Arab republic of egypt. They were housed under temperature- and light-controlled weather condition with standard laboratory rodent chow and water provided ad libitum. Animal procedures were canonical by the Ideals Committee of the National Inquiry Centre and were performed in compliance with the recommendations of the National Institutes of Health Guide for Care and Employ of Laboratory Animals (publication no. 85-23, revised 1985).
Chemicals
Reserpine (Mallinckrodlt. Inc., Martin Luther King Jr. Blvd, Paris-Kantucky) was dissolved in glacial acetic acrid (ane μg/μl) and and then completed to 25 ml with distilled water. Caffeine was purchased from Global Chemie (Mombai, Bharat). It was dissolved in 0.nine% saline (15 mg/ml). All other reagents were obtained from Sigma Chemical Co. (St. Louis MO, United states).
Experimental design
At the beginning of the experiment, the rats were divided randomly into control rats that received the vehicle till the end of experiment and reserpine-treated rats that were injected intraperitoneally (i.p.) with reserpine (0.2 mg/kg/day) for 15 days to establish the animal model of low according to Antkiewicz-Michaluk et al. (2014). On the 16th twenty-four hour period, the reserpine-treated rats were further divided into two groups: rat model of depression that continued receiving an i.p. injection of reserpine (0.i mg/kg/day) for further 15 days to keep the state of low and rat model of depression in which rats were treated daily with an i.p. injection of reserpine (0.ane mg/kg/day) followed by an i.p. injection of caffeine (30 mg/kg) with 1-h interval between the two drugs for 15 days.
Behavioral assay
The open field test was used at the end of the experiment for the control, depressed rats, and depressed rats treated with caffeine to screen the impairment in motor activity.
The open up field test
The open up field appliance was constructed of white plywood and measured 72 × 72 cm with 36 cm walls. One of the walls was clear Plexiglas, then rats could be visible in the apparatus. Blue lines were drawn on the floor with a mark and were visible through the clear Plexiglas floor. The lines divided the floor into sixteen eighteen × 18 cm squares. A central foursquare (18 cm × 18 cm) was fatigued in the middle of the open field. Rats were placed individually in the middle of the open up-field and behavioral parameters were assessed manually for x min. Five motor parameters were quantified throughout this test: central square duration (the duration of fourth dimension the rats spent in the central foursquare), line crossings (the number of times the rats crossed 1 of the filigree lines with all iv paws), rearing (the number of times the rats stood on their hind legs in the maze), freezing fourth dimension (duration in which the rat was completely stationary), and grooming (number of body-cleaning with paws, licking of the body and pubis with the mouth, and face-washing actions). The open field apparatus was cleaned after each session using seventy% ethyl alcohol and permitted to dry between tests (Brown et al. 1999).
Post-obit behavioral testing, the animals were sacrificed past sudden decapitation. The brain of each brute was speedily removed and rapidly transferred to an ice-cold Petri dish. Each brain was divided into 2 halves; right and left. Each half was dissected to obtain the cortex and hippocampus. The right half of each rat was used to measure the oxidative stress parameters and enzyme activities. The left one-half was used to measure serotonin, norepinephrine, and dopamine neurotransmitters. Each brain expanse was weighed and frozen at − lxxx °C until analyzed.
Neurotransmitter analysis
The right halves of each cortex and hippocampus were homogenized in an ice-cold solution of acidified north-butanol. The homogenates were centrifuged at 2000 rpm for 5 min. The supernatants were used for the interpretation of dopamine (DA), norepinephrine (NE), and serotonin (v-hydroxytryptamine; 5-HT) according to the fluorometric method described by Ciarlone (Ciarlone 1978). The fluorescence was measured using a spectrofluorometer (model Jasco-FP-6500, Nippon) with a source of xenon arc lamp 150 West.
Analysis of oxidative stress parameters and enzymes' activities
The left halves of each cortex and hippocampus were homogenized in Tris-HCl buffer (pH 7.iv). The homogenate was centrifuged at 5000 rpm and 4 °C for ten min. The supernatant was stored at − 70 °C until analysis. This supernatant was used to clarify the following parameters.
Determination of lipid peroxidation
Malondialdehyde (MDA), a measure of membrane lipid peroxidation, was estimated according to the method of Ruiz-Larrea et al. (1994). MDA was adamant past measuring thiobarbituric reactive species (TBARS). Ane molecule of MDA reacts with two molecules of thiobarbituric acrid in acidic medium at temperature of 95 °C for 20 min to form thiobarbituric acid reactive substances; the absorbance of the resultant pink product was measured at 532 nm using UV-visible spectrophotometer (model UV-2401 PC, Shimadzu, Japan).
Determination of nitric oxide
Nitric oxide (NO) was determined spectrophotometrically in the encephalon tissue according to the method described by Moshage et al. (1995). This method is based on the measurement of endogenous nitrite concentration as an indicator of nitric oxide production. It depends on the addition of Griess reagent which converts nitrite into a deep purple azo compound whose absorbance is read at 540 nm.
Determination of reduced glutathione
Reduced glutathione (GSH) level in the selected brain regions was determined by the method of Moron et al. (1979). This method is based on the reduction of v,five′ dithiobis-(2-nitrobenzoic acid) or DTNB "Ellman's reagent" with GSH to produce a yellow compound. The reduced chromogen is straight proportional to GSH concentration and its absorbance is measured at 412 nm. Total GSH content was expressed in mmol/1000 brain tissue.
Determination of acetylcholinesterase activeness
Acetylcholinesterase (Anguish) activity was measured according to the modified method of Gorun et al. (1978). The principle of the method depends on the hydrolysis of acetylthiocholine iodide by acetylcholinesterase to produce thiocholine. Thiocholine is immune to react with the -SH reagent v,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), which is reduced to thionitrobenzoic acrid, a yellowish colored anion whose absorption is read spectrophotometrically at 412 nm. The results were expressed as μmol SH/min/grand encephalon tissue.
Determination of Na+, K+, ATPase activeness
Na+, Grand+, ATPase activity was measured spectrophotometrically according to Tsakiris et al. (2000). Na+, K+, ATPase activeness was calculated as the difference betwixt total ATPase action (Na+, M+, ATPase, and Mg-ATPase activity) and Mg-ATPase activeness.
Statistical assay
The data were expressed as ways ± Due south.E.M. Statistical significance between the groups under investigation was tested by i-way analysis of variance (ANOVA) using Statistical Package for Social Sciences (SPSS) program followed past Duncan as post-hoc test to compare the significance between groups. The difference was considered significant at p value < 0.05.
Results
Neurochemical results
In the cortex of rat model of depression, a significant decrease in the levels of serotonin (− 55.3%) and norepinephrine (− 14.6%) was recorded in comparison to control values. In the hippocampus, there was a significant decrease in serotonin, norepinephrine, and dopamine recording − 48.2%, − 45.9%, and − 35.v%, in comparison to command animals. When the depressive-similar rats were treated daily with caffeine, information technology failed to restore the level of cortical serotonin but elevated the reduced level of norepinephrine to control-similar value. In the hippocampus, caffeine improved the levels of serotonin, norepinephrine, and dopamine to nonsignificant changes as compared to command value (Table 1).
The present findings showed that cortical and hippocampal Anguish action increased significantly in rat model of depression recording 114.1% and 83.3% above the control values, respectively. Similarly, cortical and hippocampal Na+, K+, ATPase activity increased significantly (37.vi% and 30.02%, respectively) in reserpine-treated rats. Although caffeine reduced the cortical and hippocampal Anguish activity to 66.two% and 51.9%, respectively, its value still showed a significant increase above than the control value. However, caffeine treatment normalized cortical and hippocampal Na+, Yard+, ATPase activities (Tabular array 2).
In the depressive-like rat model induced past reserpine, the lipid peroxidation level increased significantly in the cortex (41.9%) and hippocampus (78.three%) as compared to control values. This increase returned to control-like values after daily caffeine handling. Notwithstanding, caffeine treatment failed to foreclose the significant increase in cortical and hippocampal nitric oxide levels induced by reserpine. The pregnant subtract in GSH level that was observed in the hippocampus of rat model of depression returned to a command-like value afterward caffeine handling. In the cortex, GSH level increased significantly afterward caffeine treatment (Table three).
Behavioral results
As demonstrated in (Fig. one), the animals representing the depression model exhibited a significant reduction in the crossed lines, rearings, and grooming. In addition, significant increases in the time spent in the primal square and freezing time were recorded in the animal model of depression when compared to command rats. Unfortunately, caffeine failed to induce whatsoever improvement in the open field parameters except for the grooming beliefs that was reversed to a meaning increase.
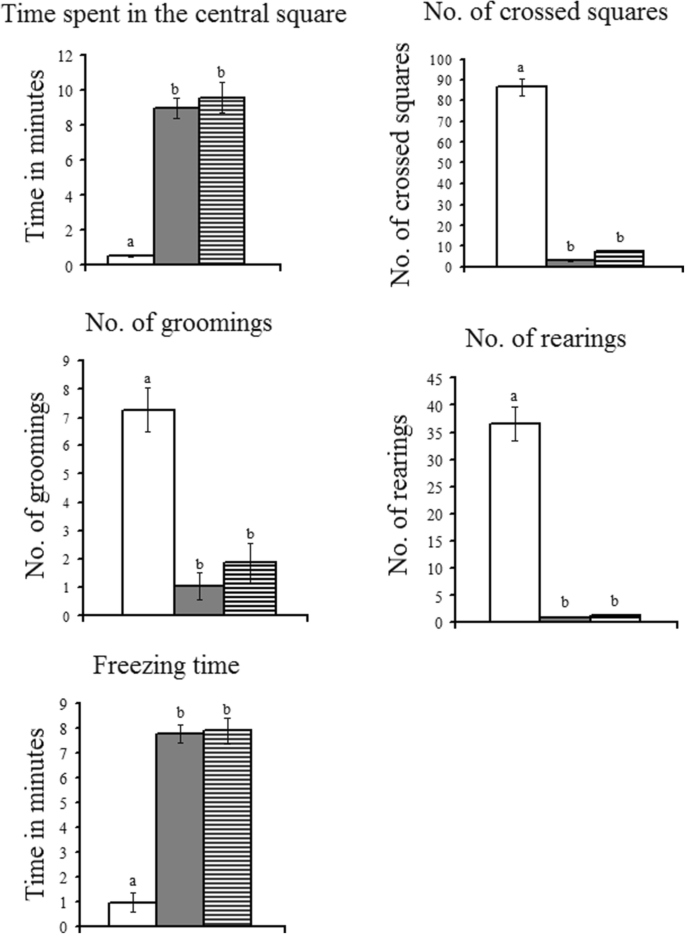
Effect of daily caffeine treatment (30 mg/kg) on the open up field test parameters in rat model of depression induced by reserpine.  command.
command.  rat model of depression.
rat model of depression.  rat model of depression treated with caffeine. Statistically significant means (p value < 0.05) are given unlike letters. Nonsignificant means are given the same letters
rat model of depression treated with caffeine. Statistically significant means (p value < 0.05) are given unlike letters. Nonsignificant means are given the same letters
Discussion
In the current report, the reserpine-induced depressive-like rat model was used to evaluate whether caffeine could treat depression or exaggerate it.
The present data revealed that the daily reserpine treatment for 30 days induced a significant decrease in cortical and hippocampal serotonin, norepinephrine and dopamine levels. In add-on, a decrease in motor activeness was observed. This was axiomatic from the data of the open field exam that exhibited significant decreases in the number of line crossings, rearings, and groomings and significant increases in the time spent in the central square and freezing time.
Immobility is an indicator of hopeless feelings and despair (Holmes 2003). Depression and immobility are proposed to be correlated with each other and it has already been shown that antidepressant drugs reduce immobility (Gersner et al. 2009).
Several studies suggested that the depletion of cerebral monoamines represents the main crusade for the pathogenesis of depression (Dale et al. 2015). Supporting this proposition, the activity of current antidepressants depends mainly on elevating levels of monoamines specially serotonin by selective serotonin reuptake inhibitors (Jakobsen et al. 2017) or monoamine oxidase inhibitors (Youdim et al. 2006). Therefore, the significant decrease in monoamines associated with the decrease in motor activity induced by reserpine indicates the evolution of rat model of depression. This model was used successfully by many studies to investigate the antidepressant upshot of many agents (Antkiewicz-Michaluk et al. 2014). Reserpine could deplete monoamines by inhibiting the activity of vesicular monoamine transporter-2 (VMAT-ii) (Erickson et al. 1992). This effect prevents the reuptake of monoamines into the synaptic vesicles exposing them to oxidative catabolism by the cytosolic enzyme monoamine oxidase (Antkiewicz-Michaluk et al. 2014). This mechanism could explain the decreased monoamine levels reported in the present study.
Equally a result to the oxidative catabolism of cytosolic dopamine, norepinephrine, and serotonin past monoamine oxidase, the cellular oxidant, hydrogen peroxide is produced (Youdim et al. 2006). In addition, monoamines, specially dopamine and norepinephrine, can undergo spontaneous cytoplasmic oxidation leading to the destruction of cellular structures (Wasik et al. 2009). These reactions produce several potentially neurotoxic byproducts, such as hydrogen peroxide (Barros-Miñones et al. 2015). Hydrogen peroxide can result in the generation of reactive oxygen species and induce neuronal apoptosis due to mitochondrial damage (Bortolato et al. 2008). The present findings revealed a state of oxidative stress after reserpine treatment in both the cortex and hippocampus. This was axiomatic from the significantly increased lipid peroxidation (MDA) and nitric oxide (NO) levels together with the significantly decreased reduced glutathione (GSH). Nether such weather of inhibition of VMAT-2 induced by reserpine and the effect of monamine oxidase, the oxidative catabolism may explain the present oxidative stress observed in the cortex and hippocampus of reserpine-induced rat model of depression. Moreover, the observed increased MDA levels may arise from the attack of the neuronal membrane phospholipids past free radicals produced from monoamine catabolism. Malondialdehyde is produced from decomposition of products of lipid peroxidation (Gaweł et al. 2004). In addition, the cortical and hippocampal increase in NO may be mediated past the increased inducible nitric oxide synthase (iNOS) and neuronal NOS expression that have been reported in depressed rats and rats subjected to immobilization stress (Gałecki et al. 2012; Maes et al. 2011).
Our results are in agreement with several studies which showed that oxidative stress is a contributing gene in the pathogenesis of psychiatric disease including depression to the extent that antioxidants have been suggested every bit neuroprotectors against depression (Behr and Moreira 2012). Moreover, oxidative stress may stand for the mechanism underlying the neurodegeneration observed in low and may explain the reduced volume of hippocampus (Arnone et al., 2012).
Charles et al. (1994) plant an increment in choline, the rate-limiting precursor in the synthesis of acetylcholine in the brains of depressed patients compared to normal controls. Higley and Picciotto (2014) reported that the enhancement of ACh signaling can induce depressive symptoms in humans and animal models. Using homo imaging, Saricicek et al. (2012) suggested that the levels of ACh are increased in patients with agile low, every bit assessed by the occupation of nicotinic receptors all over the brain, and remain elevated in patients with a history of low. This may support the hypothesis relating adrenergic-cholinergic balance with low and mania (Janowsky et al. 1972). This hypothesis suggests that elevated cholinergic tone and reduced noradrenergic tone may cause depressive symptoms and has been supported past several lines of bear witness from rodent and homo studies (Mineur et al. 2013). Several selective monoamine reuptake inhibitors have been characterized as nicotinic ACh receptor antagonists (Fryer and Lukas 1999; Hennings et al. 1999). AchE is the enzyme responsible for catabolism of ACh to cease its action. Therefore, the increased Ache activity observed in the present study may be attributed to the increased ACh, AchE substrate. As well, this increase in AchE may correspond a compensatory mechanism to attenuate the increase in cholinergic activity that has been reported in depression.
The increase of neuronal excitability in the CNS can consume much of the energy of the cells. It has been estimated that more than one half of the total ATP produced in the encephalon at residuum is used upwards past Na+, Grand+, ATPase to maintain the proper ionic gradients across the jail cell membrane (Erecińska and Silver 1989). Na+, Grand+, ATPase activeness acts to restore the membrane action potential past the efflux of Na ions and influx of K ions (Albers and Siegel 2012). Therefore, decreased Na+, K+, ATPase action has been reported with increased excitability (Souza et al. 2013). This is due to its exhaustion in restoring ionic gradients beyond the cell membrane. Thus, under the nowadays atmospheric condition of low and reduced neuronal excitability, the increase in the activity of Na+, G+, ATPase was expected.
The present findings betoken that the decrease in cortical serotonin level induced in rat model of depression connected later caffeine treatment. Even so, caffeine restored cortical norepinephrine and dopamine to command-like values. In the hippocampus, caffeine treatment resulted in nonsignificant increases in serotonin, norepinephrine, and dopamine levels. Information technology is clear that caffeine has a partial antidepressant outcome.
It has been reported that caffeine has a stimulatory effect on tyrosine hydroxylase, the charge per unit-limiting enzyme in catecholamine synthesis (Hsu et al. 2010) and an inhibitory effect on monoamine oxidase enzyme, the monoamines catabolizing enzyme (Akomolafe et al. 2017). These effects of caffeine could explain the recovery in cortical and hippocampal norepinephrine and dopamine. Moreover, the inhibitory upshot of caffeine on monamine oxidase activity may underlie the amelioration in hippocampal serotonin level. The nowadays information is supported by the study of Fredholm (1995) who constitute that caffeine can drag the reduced levels of monoamine neurotransmitters recorded in depressive disorders, an effect mediated past antagonizing A1 adenosine receptor. In improver, one of the important steps toward the treatment of depression is the stimulatory effect of caffeine on the release of serotonin in the limbic brain regions and dopamine in the prefrontal cerebral cortex. This effect of caffeine resembles the action of antidepressants (Acquas et al. 2002; Fredholm 1995). Accordingly, the present increment in dopamine especially in the hippocampus may take a role in improving mood and the land of the depressive-like model. Dopamine is the neurotransmitter involved in regulation of mood and sense of well-being. In add-on, the stimulatory upshot of caffeine on the release of neurotransmitters including dopamine, norepinephrine, and serotonin may have a role in increasing the monoaminergic activity which is reduced in low.
Although caffeine treatment failed to better the increased AchE action induced in rat model of low, there is a partial improvement in AchE activeness from 114.1% and 83.three% to 66.two% and 51.9% in the cortex and hippocampus, respectively. Akomolafe et al. (2017) recorded an inhibitory effect of caffeine on Anguish. This may be an indicator of the inability of caffeine to restore the cholinergic action to its normal value.
The antioxidant activity of caffeine has been demonstrated (Aoyama et al. 2011). This issue has been attributed to its action as a ROS scavenger specially for the hydroxyl radicals (OH−) (Devasagayam et al. 1996; Shi et al. 1991). Additionally, caffeine has been shown to prevent Fenton's reaction-induced oxidation of GSH (Shi et al. 1991), a major nonenzymatic antioxidant reserve in many tissues, including the CNS. Thus, the antioxidant effect of caffeine obtained in the present study may be attributed to its inhibitory effect on monoamine oxidase which in turn may prevent the oxidative catabolism and germination of free radicals. The nowadays ameliorating effect of caffeine on GSH may be mediated by the increase in GSH synthesis (Aoyama et al. 2011).
Several studies showed that caffeine significantly increased NO levels (Alasehirli et al. 2005; Hashiguchi et al. 2001). This effect could be attributed to the activation of endothelial NOS leading to the increase in NO (Cappelletti et al. 2015). Therefore, the present elevation in the levels of cortical and hippocampal NO could be due to the activation of NOS. The increased cerebral NO level may induce vasodilating effect (Toda et al. 2009) and consequently increased cerebral blood flow. This outcome may have a office in the benign issue exerted by caffeine on cognitive functions (Cunha and Agostinho 2010).
The present amelioration in Na+, Yard+, ATPase activity induced by caffeine may correspond a step toward the improvement in the depression model. The recovery in Na+, K+, ATPase activity is an indicator of returning the normal polarity of the prison cell membrane.
The behavioral data showed that caffeine maintained the changes in open field test parameters induced by reserpine (a significant decrease in number of crossed lines, rearings, and groomings and a significant increment in the time spent in central foursquare and freezing time).
Information technology has been reported by several studies that caffeine has motor-stimulating consequence (Nehlig et al. 1992). Still, caffeine in the present written report failed to induce any comeback in the deficits of motor activeness induced by reserpine. This could be attributed to the tolerance of motor stimulation that has been observed with the chronic treatment of caffeine (Holtzman and Finn 1988; Karcz-Kubicha et al. 2003; Svenningsson et al. 1999). Caffeine induced a dose-dependent anxiogenic effect in rats every bit axiomatic from the reduced rears and ambulation, and the increased immobility and defecation in the open-field test, and the increased number of entries and stay duration in the airtight artillery of the elevated plus-maze (Bhattacharya et al. 1997). Therefore, the inability of caffeine to restore the decrease in motor activity induced by reserpine, in the present study, may be attributed to the evolution of tolerance to the reported motor stimulant issue of caffeine on the i hand and the anxiogenic outcome arising from the blocking of adenosine receptors (A1and Atwo) on the other hand.
The present findings signal that caffeine improved monoamine levels, and Na+, Grand+, ATPase activeness and prevented the increased lipid peroxidation and decreased GSH in the cortex and hippocampus. In addition, the increased level of NO in the two brain regions could have a vasodilating effect. However, caffeine failed to restore the activity of Ache in the cortex and hippocampus and cortical serotonin level and the reduced motor activity.
Conclusion
The present data showed that the daily caffeine treatment for 15 days induced a fractional antidepressant effect. This upshot was mediated by the increased level of cortical and hippocampal serotonin, norepinephrine, and dopamine. In addition, the antioxidant and vasodilating effect exerted by caffeine may as well have a role in its antidepressant-like activity.
References
-
Acquas E, Tanda Grand, Di Chiara G (2002) Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology 27:182–193
-
Akomolafe SF, Akinyemi AJ, Ogunsuyi OB, Oyeleye SI, Oboh G, Adeoyo OO, Allismith YR (2017) Outcome of caffeine, caffeic acid and their various combinations on enzymes of cholinergic, monoaminergic and purinergic systems critical to neurodegeneration in rat brain-in vitro. Neurotoxicology 62:six–thirteen
-
Alasehirli B, Cekmen One thousand, Nacak M, Balat A (2005) Furnishings of caffeine on placental total nitrite concentration: a 21-day, vehicle-controlled study in rats. Curr Ther Res 66:130–137
-
Albers RW, Siegel GI (2012) Membrane send. In: Brady ST, Siegel GJ, Albers RW, Price D (eds) Basic neurochemistry: principles of molecular, cellular and medical neurobiology, 8th edn. Elsevier Academic Press, Massachusetts, The states, pp 41–62
-
Antkiewicz-Michaluk 50, Wąsik A, Możdżeń Due east, Romańska I, Michaluk J (2014) Antidepressant-like issue of tetrahydroisoquinoline amines in the animal model of depressive disorder induced by repeated administration of a low dose of reserpine: behavioral and neurochemical studies in the rat. Neurotox Res 26:85–98
-
Aoyama Grand, Matsumura N, Watabe 1000, Wang F, Kikuchi-Utsumi Thousand, Nakaki T (2011) Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience 181:206–215
-
Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM (2012) Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol 22:1–16
-
Barros-Miñones L, Goñi-Allo B, Suquia 5, Beitia K, Aguirre N, Puerta E (2015) Contribution of dopamine to mitochondrial circuitous I inhibition and dopaminergic deficits caused past methylenedioxymethamphetamine in mice. Neuropharmacology 93:124–133
-
Behr GA, Moreira JC, Frey BN (2012) Preclinical and clinical show of antioxidant furnishings of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxidative Med Prison cell Longev 2012:609421. https://doi.org/ten.1155/2012/609421
-
Bhattacharya SK, Satyan KS, Chakrabarti A (1997) Anxiogenic activeness of caffeine: an experimental written report in rats. J Psychopharmacol 11:219–224
-
Blardi P, de Lalla A, Urso R, Auteri A, Dell'Erba A, Bossini L, Castrogiovanni P (2005) Activeness of citalopram on adenosine and serotonin circulating levels in depressed patients. J Clin Psychopharmacol 25:262–266
-
Bortolato M, Chen K, Shih JC (2008) Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev 60:1527–1533
-
Brown RE, Corey SC, Moore AK (1999) Differences in measures of exploration and fearfulness in MHC-congenic C57BL/6J and B6-H-2K mice. Behav Genet 29:263–271
-
Cappelletti S, Daria P, Sani Yard, Aromatario M (2015) Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol xiii(2015):71–88
-
Charles HC, Lazeyras F, Krishnan KR, Boyko OB, Payne M, Moore D (1994) Brain choline in depression: in vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Prog. Neuropsychopharmacol. Biol Psychiatry xviii:1121–1127
-
Ciarlone AE (1978) Further modification of a fluoromertric method for analyzing encephalon amines. Microchem J 23:9–12
-
Cunha RA, Agostinho PM (2010) Chronic caffeine consumption prevents memory disturbance in different beast models of memory reject. J Alzheimers Dis 20:S95–S116
-
Dale E, Blindside-Andersen B, Sánchez C (2015) Emerging mechanisms and treatments for low beyond SSRIs and SNRIs. Biochem Pharmacol 95:81–97
-
Devasagayam TP, Kamat JP, Mohan H, Kesavan PC (1996) Caffeine as an antioxidant: inhibition of lipid peroxidation induced by reactive oxygen species. Biochim Biophys Acta 1282:63–seventy
-
El Yacoubi One thousand, Ledent C, Parmentier M, Bertorelli R, Ongini Due east, Costentin J, Vaugeois JM (2001) Adenosine A2A receptor antagonists are potential antidepressants: evidence based on pharmacology and A2A receptor knockout mice. Br J Pharmacol 134:68–77
-
Erecińska K, Silverish IA (1989) ATP and encephalon function. J Cereb Blood Flow Metab 9:2–19
-
Erickson JD, Eiden LE, Hoffman BJ (1992) Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci U South A 89:10993–10997
-
Ferré Southward (2016) Mechanisms of the psychostimulant effects of caffeine:implications for substance apply disorder. Psychopharmacology 233(ten):1963–1979
-
Ferré Southward, O'Connor WT, Svenningsson P, Björklund L, Lindberg J, Tinner B, Strömberg I, Goldstein M, Ögren So, Ungerstedt U, Fredholm BB, Fuxe K (1996a) Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci 8:1545–1553
-
Ferré Due south, Popoli P, Tinner-Staines B, Fuxe 1000 (1996b) Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist bounden sites. Neurosci Lett 208:109–112
-
Fisone G, Borgkvist A, Usiello A (2004) Caffeine every bit a psychomotor stimulant: mechanism of action. Cell Mol Life Sci 61:857–872
-
Fredholm BB (1995) Astra award lecture. Adenosine, adenosine receptorsand the actions of caffeine. Pharmacol Toxicol 76:93–101
-
Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51:83–133
-
Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM (2005) Adenosine and encephalon function. Int Rev Neurobiol 63:191–270
-
Fryer JD, Lukas RJ (1999) Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J Neurochem 72:1117–1124
-
Gałecki P, Gałecka E, Maes M, Chamielec Yard, Orzechowska A, Bobińska Chiliad, Lewiński A, Szemraj J (2012) The expression of genes encoding for COX-two, MPO, iNOS, and sPLA2-IIA in patients with recurrent depressive disorder. J Affect Disord 138:360–366
-
Garrett Be, Griffiths RR (1997) The role of dopamine in the behavioral furnishings of caffeine in animals and humans. Pharmacol Biochem Behav 53:533–541
-
Gaweł Due south, Wardas K, Niedworok E, Wardas P (2004) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek 57:453–455
-
Gersner R, Gordon-Kiwkowitz Thousand, Zangen A (2009) Automatic behavioral analysis of limbs' activeness in the forced swim examination. J Neurosci Methods 180:82–86
-
Gorun V, Proinov I, Baltescu V, Balaban 1000, Barzu O (1978) Modified Ellman procedure for assay of cholinesterases in rough enzymatic preparation. Anal Biochem 86(one):324–326
-
Guo Ten, Park Y, Freedman ND, Sinha R, Hollenbeck AR, Blair A, Chen H (2014) Sweetened beverages, java, and tea and depression gamble among older Usa adults. PLoS One 9:e94715
-
Hashiguchi Due west, Nagatomo I, Akasaki Y, Uchida M, Tominaga M, Takigawa M (2001) Influences of caffeine to nitric oxide product and zonisamide concentration in the encephalon of seizure susceptible EL mice. Psychiatry Clin Neurosci 55:319–324
-
Hennings EC, Kiss JP, De Oliveira Yard, Toth PT, Vizi ES (1999) Nicotinic acetylcholine receptor antagonistic activeness of monoamine uptake blockers in rat hippocampal slices. J Neurochem 73:1043–1050
-
Higley MJ, Picciotto MR (2014) Neuromodulation by acetylcholine: examples from schizophrenia and depression. Curr Opin Neurobiol 29:88–95
-
Holmes PV (2003) Rodent models of depression: reexamining validity without anthropomorphic inference. Crit Rev Neurobiol 15:143–174
-
Holtzman SG, Finn IB (1988) Tolerance to behavioral effects of caffeine in rats. Pharmacol Biochem Behav 29:411–418
-
Hsu CW, Wang CS, Chiu Thursday (2010) Caffeine and a selective adenosine A2A receptor antagonist induce sensitization and cantankerous-sensitization behavior associated with increased striatal dopamine in mice. J Biomed Sci 17:4. https://doi.org/10.1186/1423-0127-17-four
-
Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller Grand, Iversen M, Banke MB, Petersen IJ, Klingenberg SL, Krogh J, Ebert SE, Timm A, Lindschou J, Gluud C (2017) Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry 17:162. https://doi.org/10.1186/s12888-017-1311-5
-
Janowsky DS, el- Yousef MK, Davis JM, Sekerke HJ (1972) A cholinergic-adrenergic hypothesis of mania and depression. Lancet 2:632–635
-
Jin MJ, Yoon CH, Ko HJ, Kim HM, Kim AS, Moon HN, Jung SP (2016) The relationship of caffeine intake with low, feet, stress, and sleep in Korean adolescents. Korean J Fam Med 37:111–116
-
Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas Grand, Justinova Z, Pezzola A, Reggio R, Müller CE, Fuxe Yard, Goldberg SR, Popoli P, Ferré South (2003) Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine afterwards its acute and chronic administration. Neuropsychopharmacology 28:1281–1291
-
Kuzmin A, Johansson B, Gimenez L, Ögren S, Fredholm BB (2006) Combination of adenosine A1 and A2A receptor blocking agents induces caffeine-like locomotor stimulation in mice. Eur Neuropsychopharmacol 16:129–136
-
Maes M, Galecki P, Chang YS, Berk One thousand (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog Neuro-Psychopharmacol Biol Psychiatry 35:676–692
-
Mathers CD, Loncar D (2006) Projections of global mortality and brunt of affliction from 2002 to 2030. PLoS Med three:e442
-
Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR (2013) Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like beliefs. Proc Natl Acad Sci U S A 110:3573–3578
-
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta 582:67–67
-
Moshage H, Kok B, Huizenga JR, Jansen PL (1995) Nitrite and nitrate decision in plasma: a critical evaluation. Clin Chem 41(vi Pt 1):892–896
-
Nehlig A, Daval J-Fifty, Debry G (1992) Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Encephalon Res Rev 17:139–170
-
Pham NM, Nanri A, Kurotani K, Kuwahara Thou, Kume A, Sato M, Hayabuchi H, Mizoue T (2014) Green tea and coffee consumption is inversely associated with depressive symptoms in a Japanese working population. Public Wellness Nutr 17:625–633
-
Porkka-Heiskanen T (1999) Adenosine in slumber and wakefulness. Ann Med 31:125–129
-
Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H (1994) Antioxidant effects of estradiol and 2-hydroxyestradiol on atomic number 26-induced lipid peroxidation of rat liver microsomes. Steroids 59:383–388
-
Ruusunen A, Lehto SM, Tolmunen T, Mursu J, Kaplan GA, Voutilainen S (2010) Coffee, tea and caffeine intake and the chance of severe depression in middle-aged Finnish men: the Kuopio ischaemic heart illness run a risk cistron study. Public Wellness Nutr xiii:1215–1220
-
Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, Tamminga CA, Pittman B, Bois F, Tamagnan G, Seibyl J, Picciotto MR, Staley JK, Bhagwagar Z (2012) Persistent β2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry 169:851–859
-
Schechter LE, Ring RH, Bayer CE, Haghes ZA, Khawaja Ten, Malberg JE, Rosenzweig-Lipson Due south (2005) Innovative approaches for the development of antidepressant drugs: current and future strategies. J Am Soc Exp Neurotherap 2:590–611
-
Shi X, Dalal NS, Jain Ac (1991) Antioxidant behaviour of caffeine: efficient scavenging of hydroxyl radicals. Food Chem Toxicol 29:1–6
-
Souza MA, Mota BC, Gerbatin RR, Rodrigues FS, Castro Yard, Fighera MR, Royes LF (2013) Antioxidant activity elicited by low dose of caffeine attenuates pentylenetetrazol-induced seizures and oxidative damage in rats. Neurochem Int 62:821–830
-
Svenningsson P, Nomikos GG, Fredholm BB (1999) The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci 19:4011–4022
-
Toda N, Ayajiki K, Okamura T (2009) Cognitive claret flow regulation by nitric oxide in neurological disorders. Can J Physiol Pharmacol 87:581–594
-
Tsakiris Southward, Angelogianni P, Schulpis KH, Behrakis P (2000) Protective effect of 50-cysteine and glutathione on rat brain Na+, Chiliad+ ATPase inhibition induced by complimentary radicals. Z Naturforsch 55:271–277
-
Wang L, Shen X, Wu Y, Zhang D (2016) Java and caffeine consumption and depression: A meta-assay of observational studies. Aust NZJ Psychiatry 50:228–242
-
Wasik A, Romańska I, Antkiewicz-Michaluk L (2009) 1-Benzyl-1,ii,3,iv-tetrahydroisoquinoline, an endogenous parkinsonism-inducing toxin, strongly potentiates MAO-dependent dopamine oxidation and impairs dopamine release: ex vivo and in vivo neurochemical studies. Neurotox Res xv:15–23
-
Williams M (1987) Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol 27:315–345
-
Youdim MB, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Acknowledgements
Not applicable.
Funding
Non applicable.
Availability of information and materials
The datasets obtained in the present study could be requested from the corresponding author.
Author data
Affiliations
Contributions
YA Khadrawy suggested the thought, analyzed the results, and shared in writing the manuscript. HG Sawei shared in following the animals and collecting the information. EN Hosny contributed in following the animals, collecting the data, and statistical analysis. HH Mourad contributed in collecting the data and preparing the figures and tables. All the authors contributed in writing the manuscript. All authors read and approved the final manuscript.
Respective author
Ethics declarations
Ethics blessing and consent to participate
This work has been canonical past the Ethics Commission of the National Research Middle (Number: 17131) and were performed in compliance with the recommendations of the National Institutes of Health Guide for Care and Employ of Laboratory Animals (publication no. 85-23, revised 1985).
Consent for publication
Not applicative.
Competing interests
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution iv.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted utilize, distribution, and reproduction in any medium, provided you requite appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and signal if changes were made.
Reprints and Permissions
About this article
Cite this article
Khadrawy, Y.A., Sawie, H.Chiliad., Hosny, Eastward.Northward. et al. Assessment of the antidepressant effect of caffeine using rat model of low induced by reserpine. Bull Natl Res Cent 42, 36 (2018). https://doi.org/10.1186/s42269-018-0034-1
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s42269-018-0034-1
Keywords
- Depression
- Caffeine
- Monoamines
- Oxidative stress
- Acetylcholinesterase
Source: https://bnrc.springeropen.com/articles/10.1186/s42269-018-0034-1
0 Response to "Effect of Caffeine on Serotonin Levels Literature Review"
Post a Comment